GUidelines for reporting on studies
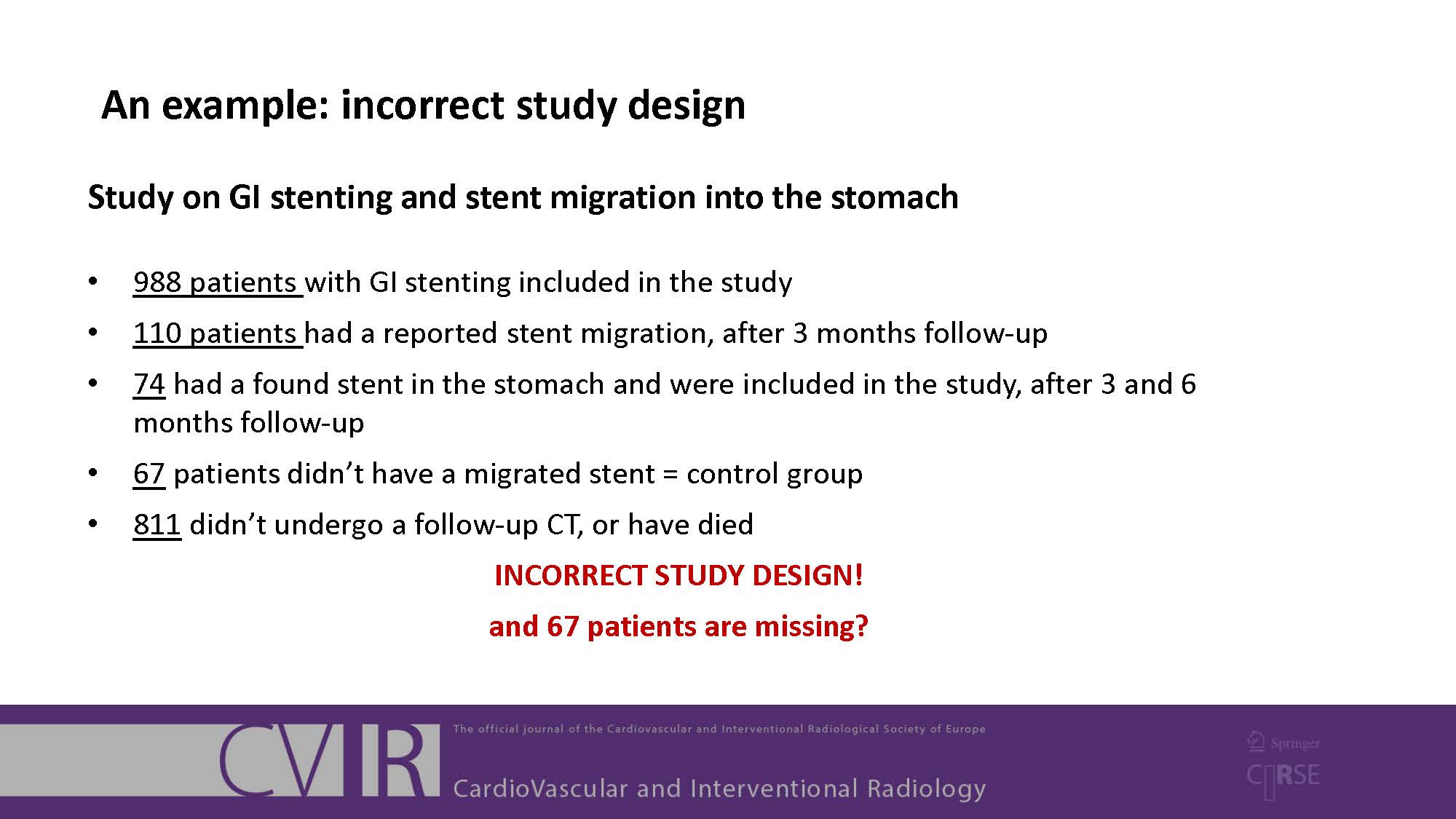
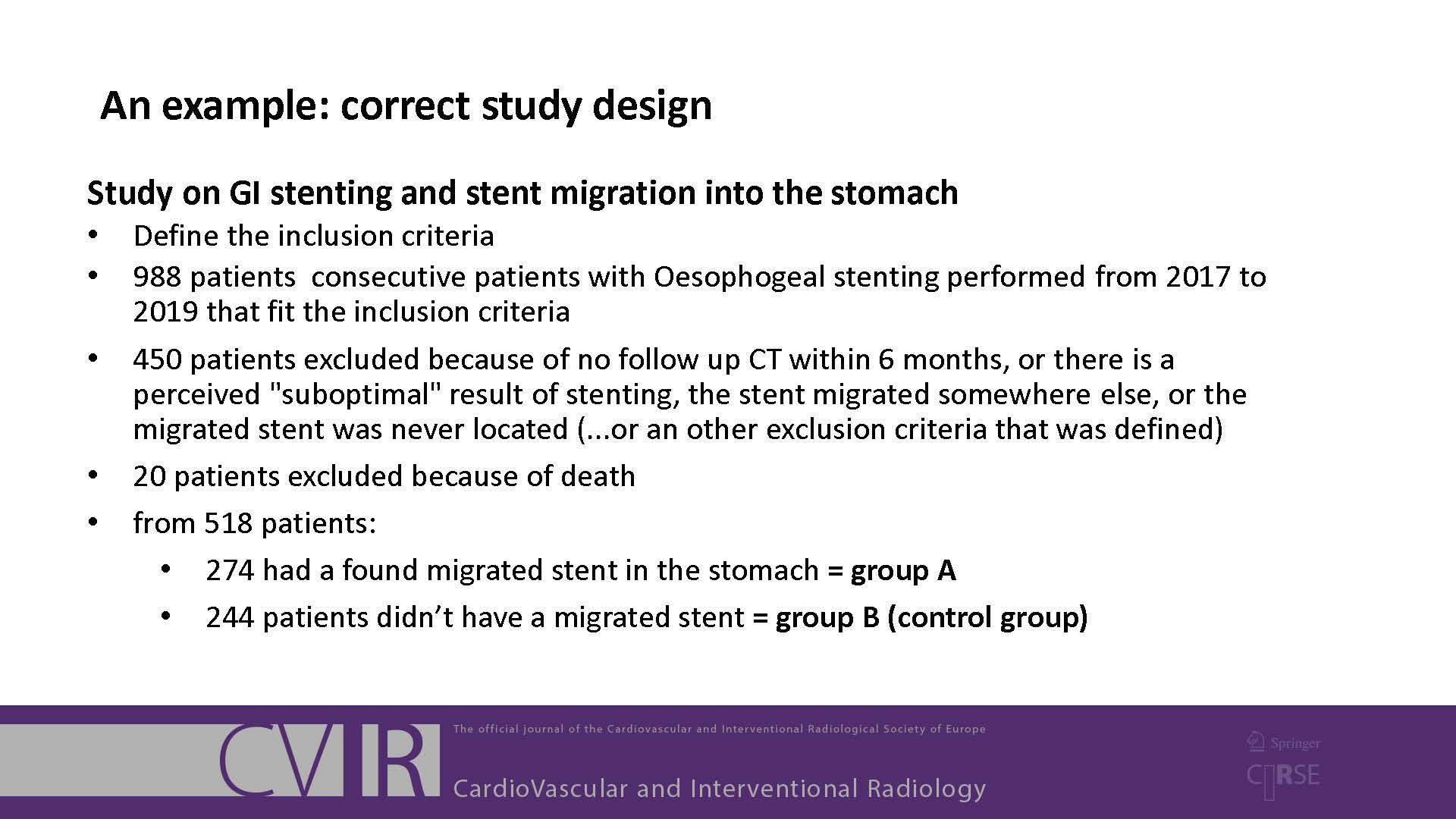
It is important that your manuscript gives a clear and complete account of the research that you have done. Well reported research is more useful and complete reporting allows editors, peer reviewers and readers to understand what you did and how.
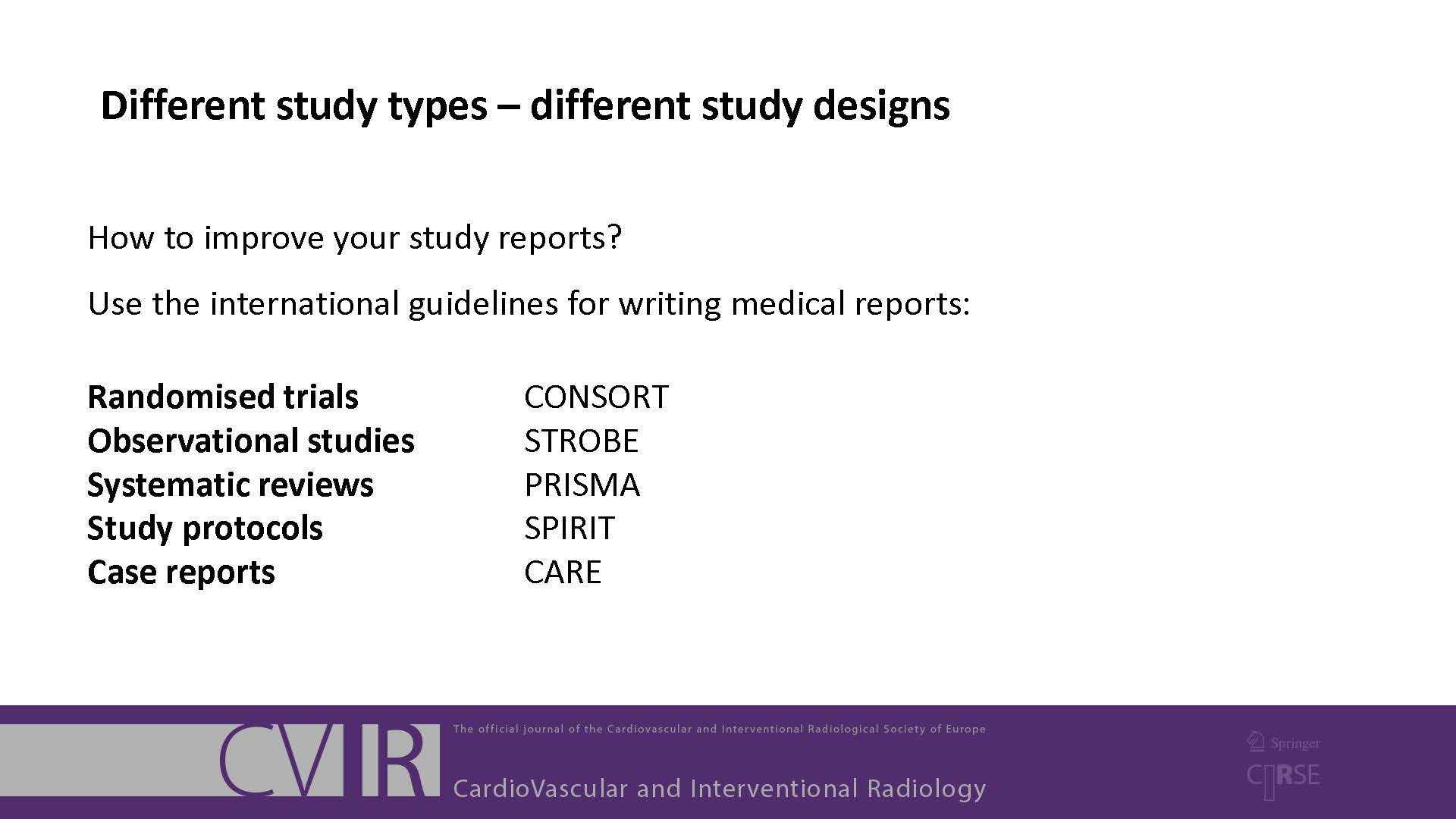
To help with reporting your research, there are reporting guidelines available for many different study designs. These contain a checklist of minimum points that you should cover in your manuscript. You should use these guidelines when you are preparing and writing your manuscript. Note that Springer has now made the following five biological/biomedical reporting checklists mandatory:
- Randomized controlled trials: CONSORT
- Randomized controlled trial protocols: SPIRIT
- Systematic reviews and meta-analyses: PRISMA
- Case reports: CARE
- Preclinical animal studies: ARRIVE
The above checklists must be completed before peer review, and made available to the editors and reviewers for evaluation during peer-review.
Springer Nature continues to strongly encourage (but not require), where relevant, the use of the following checklists and reporting guidelines:
- Observational studies: STROBE
- Systematic reviews and meta-analyses protocols: PRISMA-P
- Diagnostic/prognostic studies: STARD and TRIPOD
- Clinical practice guidelines: AGREE and RIGHT
- Qualitative research: SRQR and COREQ
- Quality improvement studies: SQUIRE
- Health economic evaluations: CHEERS
For information on instructions for authors of CVIR and formatting requirements for each article type, click here.